Your Clinical Trial Failed its Objective. Now What?
So many questions. Why didn’t more subjects respond positively to treatment? Were there hidden confounding factors? The risk of efficient trials is that despite best efforts to include, exclude and randomize subjects, your study cohorts may contain imbalances. Collecting lots of data about your subjects (think wearables) is great, but how do you sift through the data to find what matters? Let us help you apply our expertise with AI and decades of experience in clinical research to your study data and discover the answers to your questions.
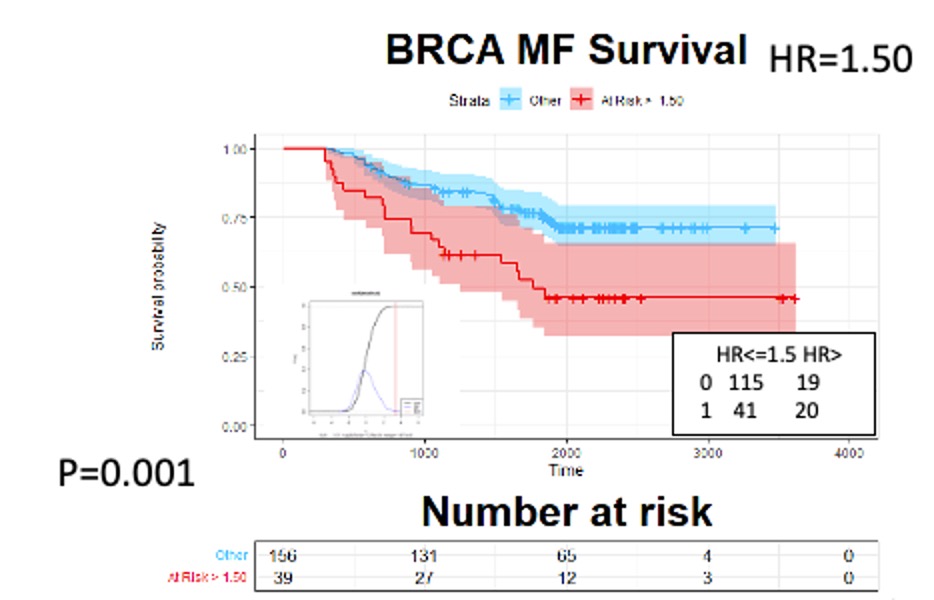
– Our analysis of a Phase 2 study revealed that the test therapy worked better for improving function and activity than reducing patient-reported pain.
– We analyzed a multi-arm study and found that a subset of non-responders obscured the statistical difference between arms.
– Selecting subjects at highest risk of fast disease progression can enable you to optimize trial resources and maximize your chance of positive outcome.
Knowing more about your therapy and past trial performance will enable you to make informed decisions about future study design, reducing risk by optimizing subject selection and outcome measures.